About Flame Arrestors and Detonation Arrestors
Flammability and Flashback Prevention (a work in progress)
Dan Banks, P.E.
Flammability:
Overview
Flammability refers to the ability of a mixture of fuel and air to sustain combustion when ignited. Hydrocarbon molecules will react with oxygen (burn) if heated sufficiently, for instance by a spark or similar ignition source. The required temperature is different for different compounds and is called the "Ignition Temperature".
The heat released by burning the hydrocarbons in the vicinity of the spark is absorbed by the hydrocarbon/air mixture nearby. If the nearby mixture picks up enough heat, it will also burn, releasing heat into the adjacent gas and resulting in burning of all of the surrounding mixture.
If the mixture contains too few hydrocarbon molecules, the released heat will be too little and burning will not progress.
If the mixture contains too few oxygen molecules, only part of the hydrocarbon molecules will be burned and again the heat released will be insufficient for burning to continue beyond the ignition source.
In laboratory testing, various pure hydrocarbons are mixed with air to form mixtures with different hydrocarbon/air ratios. As the hydrocarbon fraction is increased, the first point at which sustained burning is observed is noted as the Lower Explosive Limit (LEL). As the hydrocarbon fraction is increased, eventually the fraction of oxygen is reduced enough that sustained burning is no longer achieved. This point is the Upper Explosive Limit (UEL). Any hydrocarbon/air mixture between the LEL and the UEL will burn, while any mixture outside of this range will not burn. Testing with methane, for instance, shows that 5% (by volume) of methane in air is the LEL. Methanes UEL is 15%. Laboratory values for a few of the tested hydrocarbons is listed below. Note that several of the compounds actually require no oxygen at all for combustion (UEL = 100), indicating that a tank of the pure hydrocarbon will burn completely once ignited. The lowest LEL in this group is 1.05% for n-Heptane; if you mix 1.05% of n-Heptane with 98.95% air, it will burn. This mixture of n-Hexane, however, would not burn:
| Hydrocarbon | Formula | LEL in air (%) | UEL in air (%) | Ignition Temperature, oF |
| Methane | CH4 |
5.0 |
15.0 |
1202 |
| Ethane | C2H6 |
3.0 |
12.4 |
959 |
| Propane | C3H8 |
2.1 |
9.5 |
871 |
| n-Butane | C4H10 |
1.8 |
8.4 |
896 |
| n-Pentane | C5H12 |
1.4 |
7.8 |
878 |
| n-Hexane | C6H14 |
1.2 |
7.4 |
527 |
| n-Heptane | C7H16 |
1.05 |
6.7 |
491 |
| Dimethyl ether | C2H6O |
3.4 |
27 |
662 |
| Hydrogen | H2 |
4.0 |
75 |
1062 |
| Ethylene oxide | C2H4O |
3.6 |
100 |
804 |
| Acetylene | C2H2 |
2.5 |
100 |
581 |
Effect of temperature
Almost all published flammability values are measured using hydrocarbon/air mixtures at room temperature. If the mixture temperature is higher, the LEL is reduced. Reference #3 reports that increasing the temperature 100
oC = 180oF decreases the LEL value about 8%. The equation to use isLt = 1.02 x L x (1-7.75 x10-4 x T), where L is the laboratory value of LEL, T is the elevated temperature and Lt is the LEL at T. The UEL value increases with increase in mixture temperature, also by about 8% with 180oF increase in T.
Effect of pressure
Increasing the mixture pressure above atmospheric affects the LEL very little. The UEL, however, increases greatly. One source reports that for several saturated hydrocarbons, the UEL increases in proportion to the logarithm of the pressure. Another source reports an opposite effect for some hydrocarbons and warns that pressure effects vary according to the hydrocarbon considered.
Effect of inerts
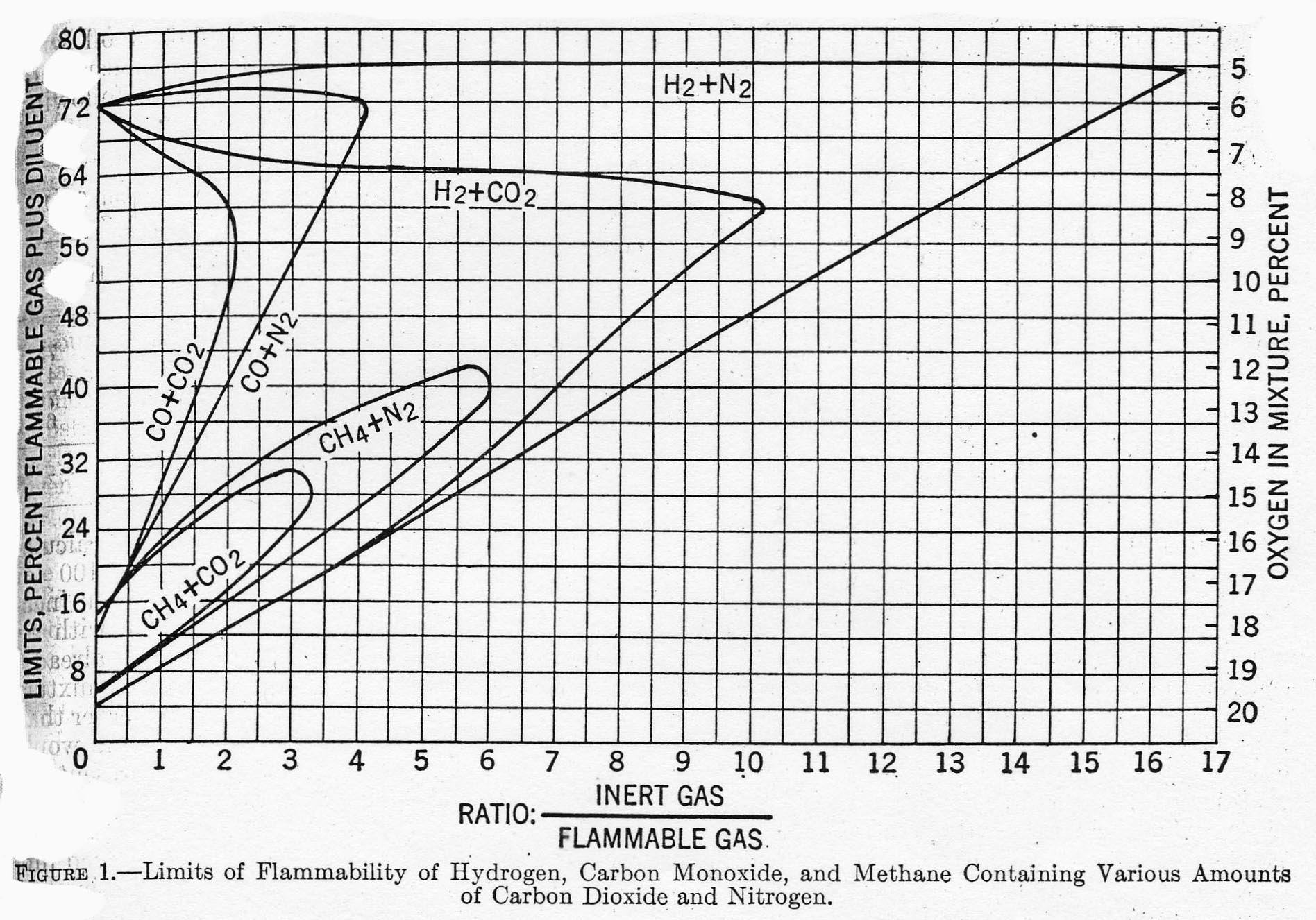
Inert gases play no part in combustion reactions, but absorb heat when present in a hydrocarbon/air mixture. For that reason, adding inerts to a mixture tends to reduce the spread between LEL and UEL until finally the mixture is no longer flammable. Reference 2 provides the graph below showing specific effects for hydrogen, carbon monoxide and methane when inerted with nitrogen and carbon monoxide. For example, methane in air with no inerts (Ratio = 0) has LEL = 5 and UEL = 15. But if 3.5 mols of CO2 are added to a mixture containing 1 mol of CH4 (in air), the mixture is no longer flammable.

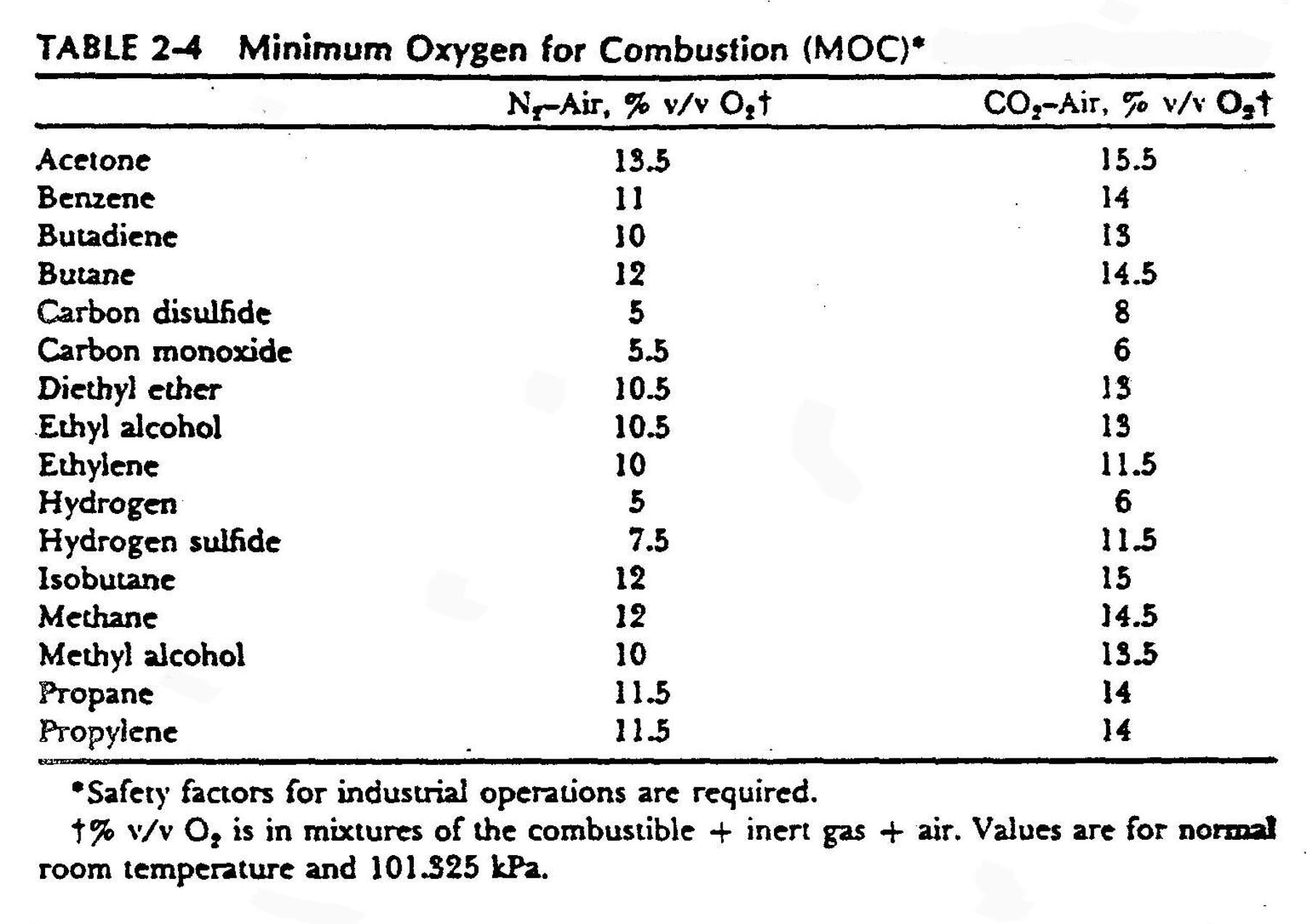
Minimum Oxygen for combustion
Another way to look at the presence of inerts is to calculate the Minimum Oxygen for Combustion (MOC). The table below from Reference 4 provides values for some compounds. The data came from Reference 5.
Flammability of hydrocarbon mixtures -
The flammability limits of a mixture of various hydrocarbons can be calculated using Le Chateliers law, which states that a mixture at the lower limit of flammability mixed with other mixtures which are also at the lower limits of flammability will yield a resulting mixture at the lower limit of flammability. For example, to calculate the LEL of a hydrocarbon mixture which is 70% CH4, 20% C2H6 and 10% C3H8,
LEL = 100% / (70/5.0 + 20/3.0 + 10/2.1) = 3.9%
This mixture has a LEL of 3.9% hydrocarbons in air. The mixture UEL is calculated the same way.
Ignition Sources:
Hot refractory
If the refractory lining a burner or furnace is hot enough to bring the hydrocarbon/air mixture to the autoignition temperature, rapid combustion will start. Heat transfer from the hot surface depends on gas velocity and turbulence, explaining why "swirl" type burners often seem more stable than more linear types.
Flame
Flames from pilot burners are the typical means of initiating combustion of a hydrocarbon/air mixture. Nozzle mix burners (where the fuel mixes with the combustion air within the furnace) have zones that are too lean or too rich for combustion, so the pilot flame must be positioned to heat a volume of well mixed gas. Large pilot flames can overcome poor positioning of the pilot tip.
Sparks
Sparks are used to ignite pilot burners and also main burners in some cases. Occasionally small sparks (static electricity) are capable of initiating combustion, but the extra energy in a large spark helps insure lightoff. Undesired sparks, such as those resulting from debris moving through steel ducting or fans, can initiate combustion and require careful design to avoid.
Flashback Prevention Methods:
Enrichment
By adding natural gas or other hydrocarbon, the mixture can be brought above the UEL, preventing combustion.
Dilution
By adding inerts such as N2 or CO2, the mixture can be brought to a nonflammable state.
Velocity
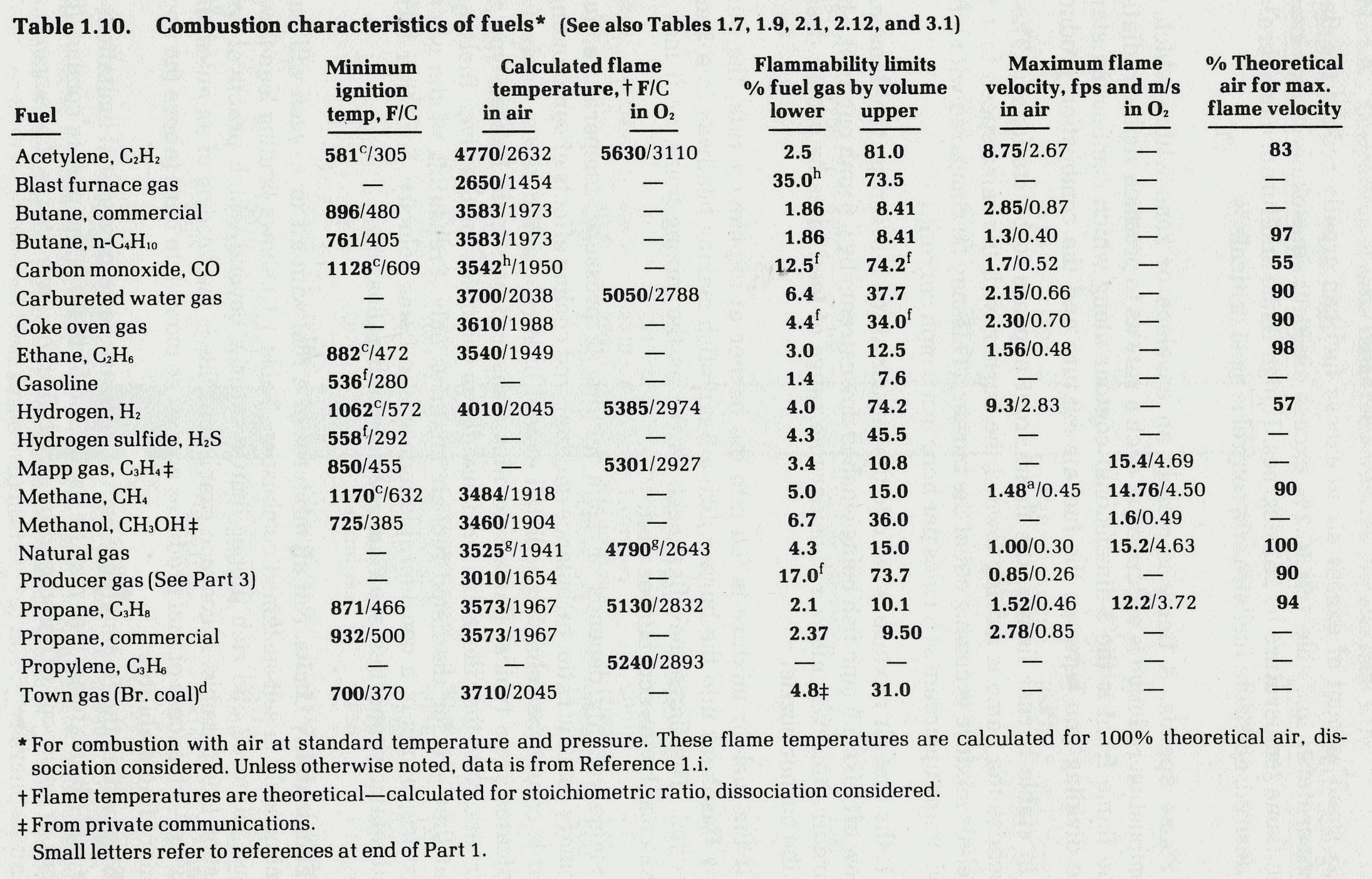
Flames progress at a defined rate through a flammable mixture. Considerable lab testing with non-turbulent mixtures has been done data for a few gases is listed below from Reference 7. For instance, the "maximum flame velocity" of a methane/air mixture is 1.48 ft/sec under lab conditions. If this mixture flows through a pipe at 1.5 ft/sec, any flame will be unable to propagate against the flow. This fact is used in designing flare tips, burner nozzle and some flame arrestors by designing for gas velocity above the flame velocity, the flame can be prevented from moving upstream from the point of ignition. NOTE: as a flame front moves through a vessel or pipe, the flame velocity increases. With long enough piping the velocity can increase to detonation levels, which are supersonic.
Flame Arrestor Tips
Mechanical flame arrestors stop flame propagation into or through a pipe (more information below). By placing a flame arrestor at the end of the flammable mixture pipe feeding a flare or burner, flame can be prevented from moving into the pipe regardless of the mixture velocity.
End-of-pipe flame arrestors
Mechanical flame arrestors may be attached to vent pipes on hydrocarbon storage tanks to allow passage of potentially flammable hydrocarbon/air mixtures but preventing passage of flame into the tank from outside. This protects storage tanks from explosions triggered by lightning ignition of vented gases outside the tank.
Cooling
For a flashback to progress into equipment, combustion heat must be transferred into the combustible mixture. By passing a potentially flammable mixture through a water spray chamber or some sort of heat sink, a flashback can be stopped. Mechanical inline flame arrestors and detonation arrestors are common heat sinks (see below).
Flashback Interruption Methods:
Many methods to stop flashbacks have been devised. "Active" methods require maintenance of certain parameters, such as liquid level or gas velocity. "Passive" methods require only routine inspection and typically have no moving parts or instrument requirements.
Venturi type flame Arrestors (active)
Venturi flame arrestors simply create a restriction in the hydrocarbon/air mixture delivery pipe so that the gas velocity is faster than the flame speed, preventing progression of a flashback upstream. Flashback in the direction of flow can still happen. Even a partly closed valve can create a high velocity for flashback prevention, but a venturi shape creates much lower pressure drop. If gas flow stops, the venturi is no longer effective, so methods to measure flow and add makeup gas (nitrogen, for instance) are often included. Note: initially flame velocity is limited to the values in the literature, but extended pipe runs and fittings act to increase the velocity, eventually reaching detonation velocities. A venturi arrestor must be located close to the point of ignition to avoid problems.
Inline flame arrestors (passive)
Mechanical flame arrestors are filled with metal or ceramic, which absorbs heat from a flashback, quenching it to a temperature below what is needed for ignition. This stops the flame. With a low enough hydrocarbon/air mixture flow rate, if a flame travels to the face of the arrestor, it can become stable at that point. Heating of the arrestor body and internals results. Once the arrestor temperatures increase enough, ignition temperature can be reached on the upstream side of the arrestor and the flashback can proceed. For this reason, a temperature switch is often installed on the flame side of each arrestor (adding an "active" element). If an elevated temperature is detected, an alarm sounds and steps can be taken to stop flow completely. An Enardo flame arrestor is shown below (
www.enardo.com).
Flame Arrestor with removable element from Enardo
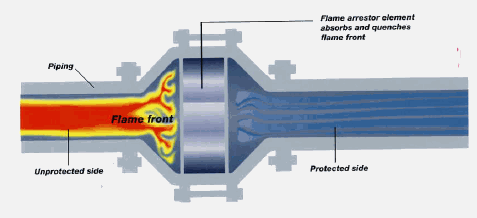
How a Flame Arrestor works
Inline detonation arrestors (passive)
Detonation arrestors are stronger, more effective versions of standard flame arrestors. They are certified after extensive testing per U.S. Coast Guard standards, which specify piping arrangements certain to accelerate a normal flash back to detonation speeds. The certified detonation arrestor must stop the flash back without damage to the arrestor itself, so it can be used repeatedly if necessary. Detonation arrestors can be certified for various hydrocarbons, which have been divided into groups according to how difficult flash backs with them are to stop. A list from Protectoseal (
www.protectoseal.com) derived from National Electric Code (NEC) Article 500 is shown below:
Detonation Arrestor Certification Classes
Group A
- acetyleneGroup B - butadiene, ethylene oxide, hydrogen, manufactured gases containing more than 30% hydrogen by volume and propylene oxide
Group C - acetaldehyde, cyclopropane, diethyl ether, ethylene, and unsymmetrical dimethyl hydrazine
Group D acetone, acrylonitrile, ammonia, benzene, butane, butyl alcohol, secondary butyl alcohol, n-butyl acetate, isobutyl acetate, ethane, ethyl alcohol, ethyl acetate, ethylene dichloride, gasoline, heptanes, hexanes, isoprene, methane (natural gas), methanol, isobutyl alcohol, methyl isobutyl ketone, isobutyl alcohol, tertiary butyl alcohol, petroleum naphtha, octanes, pentanes, amyl alcohol, propane, propyl alcohol, isopropyl alcohol, propylene, styrene, toluene, vinyl acetate, vinyl chloride, xylenes.
Note that a detonation arrestor certified for Group B hydrocarbons is also suitable for Group C and D hydrocarbons. There is some evidence that methanol belongs in Group C or B, but recertification is not complete.
Currently the European Union is defining separate standards for testing and certification of detonation arrestors acceptable in that jurisdiction.

Detonation Arrestors from Protectoseal
Liquid seal flame Arrestors (active)
This type of flame arrestor works by bubbling the hydrocarbon/air mixture upwards through a liquid bath (usually water), forming discrete bubbles. The gas exits above the liquid to the ignition source. A flash back is stopped when flame is unable to move from bubble to bubble in order to reach the upstream pipe. Some certification work has been done in Europe on this type arrestor, but so far there are no certified models on the market. A common liquid seal flame arrestor design is shown below. Note the water level must be maintained safely above the level of the sparger at all times to insure bubbles.
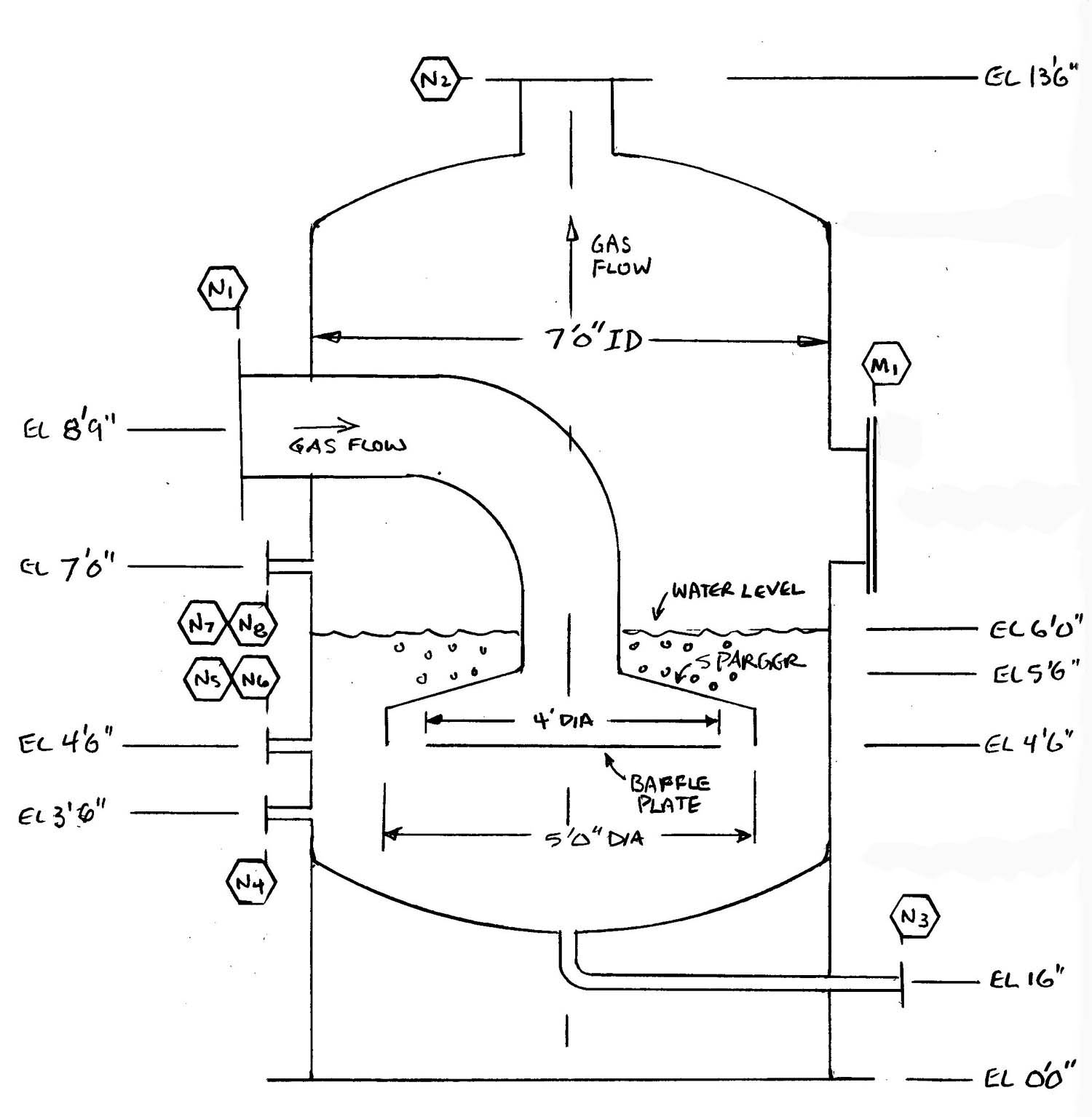
Example of a Liquid Seal Flame Arrestor
Quick acting valves (active)
By combining a very quick closing valve with very quick flame detection, Fike (
www.fike.com) and probably others have built systems which reliably stop flashbacks. The Fike valves are actuated with explosive charges similar to those used in car air bags. Each time the valve actuates, replacement of the charge (and inspection of the valve) is required. The flame detector can sense the radiation from a flame or can detect the quick pressure rise associated with a flashback, and Fike supplies a special control panel to integrate the system. Below is a Fike valve drawing from their web site: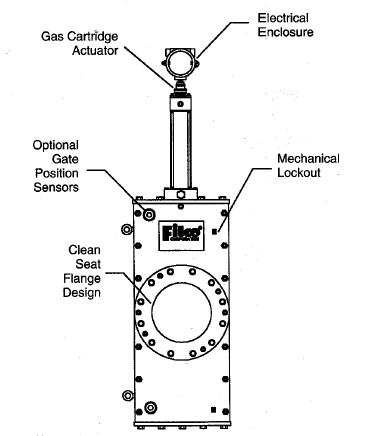
Quick Acting Flashback Prevention Valve
Inerts injection (active)
When a flash back is detected, one way of stopping it is to temporarily modify the hydrocarbon/air mixture so that it is no longer in the explosive range. Often this is done by injecting an inert gas such as nitrogen. Using this method, the mixture is diluted so that the final oxygen concentration is below the Minimum Oxygen for Combustion (defined above). This approach is sometimes used when a standing flame at a flame arrestor is detected inert gas is added upstream, snuffing the flame. The system then returns to normal operation. It can also be used for routine operation, in order to prevent the possibility of any flashbacks.
References:
1. Perrys Chemical Engineers Handbook, 4th Edition
2. Bureau of Mines Bulletin 503 "Limits of Flammability of Gases and Vapors"
3. Flammability Properties of Hydrocarbon Fuels, Wilbur A Affens, Journal of Chemical and Engineering Data, Vol. 11, No. 2, April 1966.
4. Industrial Explosion Prevention and Protection
5. National Fire Protection Association, Standard on Explosion Prevention Systems, NFPA 69, Boston, 1973.
6. Flammability Calculations for Gas Mixtures, W.M. Heffington and Gaines, W.R., Oil & Gas Journal Nov. 16, 1981.
7. North American Combustion Handbook, Second Edition.